Molecule Containing Two O Atoms: Water is made up of two atoms. The two smaller spheres in each molecule are made up of oxygen and hydrogen atoms. The hydrogen bonds that connect two water molecules are represented by dotted lines. A strong Chemical connection between hydrogen and oxygen takes a lot of energy to break. Image of Homo Enthiran (2017). A molecule is the smallest possible amount of a chemical compound. It’s the tiniest particle in an element or compound that has certain chemical properties.
https://en.wikipedia.org/wiki/Molecule
Molecules are made up of atoms that are grouped in a specific pattern. Some elements have atoms that are difficult to combine with others. The formation of a molecule is the result of chemical bonding between two or more atoms. Atoms prefer to form certain shapes over others; not all-atom combinations are created equal. The valency of the two differs as well. To give just a few examples, oxygen always has two bonds with other atoms, carbon always has four links with other atoms, and nitrogen always has three bonds with other atoms. In form of CO2, it is made up of two oxygen atoms and one carbon atom. Three atoms make up a triatomic molecule.
If you broke a molecule into tiny pieces, you wouldn’t recognize it. Two examples of these elements are oxygen and chlorine. In a diatomic atom, there can only be two atoms of the same or different elements. Diatomic molecules include oxygen and carbon dioxide. It’s a heteronuclear diatomic molecule, meaning it’s made up of two atoms from the same element. Seven diatomic elements are listed in the periodic table: Some of the most frequent elements found in the human body include oxygen, fluorine, nitrogen, iodine, and bromine (Br2). A molecule is formed when two or more atoms of the same element interact chemically.
Molecules gases such as air no permanent position
Liquid molecules, such as water molecules, are bound together but can still move. When the sorts of atoms differ from one another, a compound is formed. Because sugar is solid, its molecules can only vibrate. When atoms are ionized and molecules cannot form, plasma is the fourth state of matter. The size of a molecule is determined by the number of atoms in it. It is possible to list the atom numbers using a molecular formula. C6H12O6 is the chemical formula for glucose, for example. One molecule of glucose contains six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
Thesis assertion: “even when left alone, the tiniest atom of a substance that preserves all of its chemical features, As an illustration, consider the tiniest drop of water.” Chemical compounds are substances whose molecules contain more than one sort of element. Two examples are carbon dioxide and water. A molecular model of water is depicted. A carbon dioxide molecule is depicted here. A grouping of atomic symbols represents a molecule of an element or a compound. An example is O2O2 H2OH2O H 2 SO 4. In the molecule, two oxygen atoms are linked together. One oxygen and two hydrogens make up a molecule. The molecule is made up of two hydrogen atoms, one sulfur atom, and four oxygen atoms…
A phosphorus molecule, denoted as P4P4, is made up of four phosphorus atoms. In a single helium molecule, there is only one helium atom. The CO 2 molecule is made up of one carbon atom and two oxygen atoms. A molecule’s atomicity refers to the number of atoms it contains. Monoatomic molecules are molecules with a mass of one atom or fewer. In terms of atomicity, they’re all the same. O 2, H 2, HI, HCl, and other diatomic compounds are examples. The number of atoms in them is TWO.
The presentation’s transcript is as follows:
compound that can exist independently while retaining the chemical properties of the parent substance Water, for example, is the tiniest component of an element, yet it keeps all of the properties of the element. Consider the example of water. A water molecule is made up of one oxygen atom and two hydrogen atoms. A carbon dioxide molecule is made up of one carbon atom and two oxygen atoms. Another illustration is carbon dioxide. An ammonia molecule is made up of one nitrogen atom and three hydrogen atoms. The final example is ammonia. Every argon molecule contains only one argon atom. argon (examples 4 and 5) Most metals have atoms that look like this.
H 2 O, H 2 S, CO 2, and other compounds with three atomic atoms
They each have three atoms. A polyatomic molecule is one that has a significant number of atoms. For example, the atomicity of S 8 is EIGHTY. In regards to the many elements, The raw materials for the creation of 94 of the elements come from the Earth’s crust, oceans, and atmosphere. Others have been made up. Aluminum and carbon are two materials that we are all familiar with. They are the fundamental elements of life. Aluminum foil can be used in this situation. If the atoms of a molecule are torn apart, the substance’s properties are lost.
Characteristics of Molecules
Dividing a sample of a substance into smaller and smaller parts has no influence on its composition or chemical qualities until sections consisting of single molecules are reached. When a substance is broken down further, it creates smaller and smaller fragments, each with a distinct chemical composition from the original. At this stage of fragmentation, the bonds that hold atoms together in molecules are disrupted.
To comprehend a molecule’s relative size, we must first comprehend its molecular weight
Add the atomic weights of a molecule’s constituent atoms to find its molecular weight. Avogadro’s number (6.022140857 1023) states that the number of molecules in a mole remains constant regardless of the substance. The molecular weight of a molecule can be determined using mass spectrometry or methods based on thermodynamics or kinetic transport processes.
A molecule bond is formed when two molecules come Together
Every water molecule, for example, contains two hydrogen atoms and one oxygen atom; the number of atoms that can form molecules is fixed. Chemical compounds, in contrast to solutions and other mechanical mixtures, have this feature. Hydrogen and oxygen can be present in random amounts in mechanical combinations, but when sparked, they will only combine in particular proportions to make water (H2O). Different molecules can be created by varying the ratios of the same atoms.
Two hydrogen atoms, for example, can chemically join with one oxygen atom to form a water molecule, while two hydrogen atoms can chemically bond with two oxygen atoms to form a hydrogen peroxide molecule (H2O2). Furthermore, atoms can form new molecules with the same amount of bonding. It’s called an isomer since the sole difference between them is the arrangement of atoms in the molecules. For example, ethyl alcohol (CH3CH2OH) and methyl ether (CH3OCH3) each have one, two, or six oxygen, carbon, and hydrogen atoms, but these atoms are bonded in different ways.
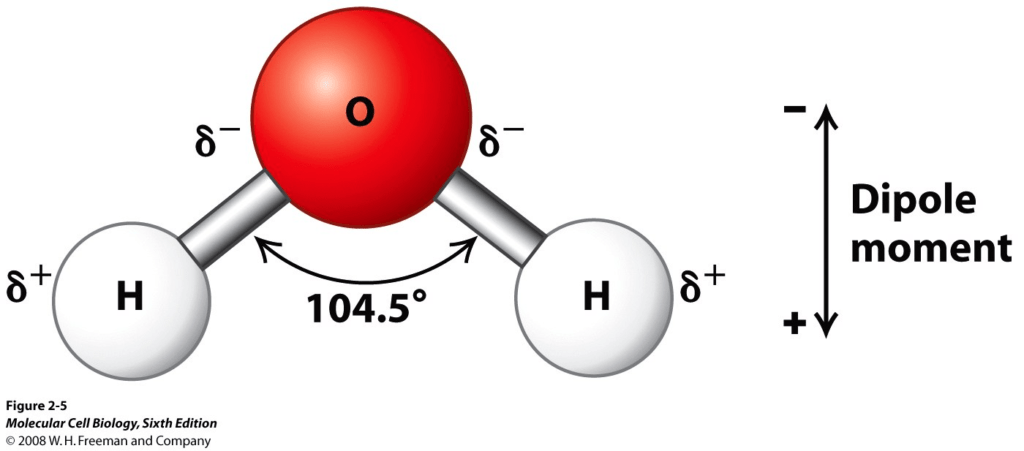
It’s crucial to comprehend the differences between polar and non-polar compounds
The negative charge is equal to the positive charge when a molecule has no net electrical charge. The arrangement of positive and negative charges in space determines the forces imposed on these molecules. The arrangement of a nonpolar molecule is spherically symmetric. Dipole moments, or a quantitative inclination to rotate in an electric or magnetic field, are found in polar molecules. This means they have a positive charge surplus on one end and a negative charge excess on the other. When they are free to do so, rotating polar molecules prefer orientations that result in attractive forces.




