Hydrocarbons Are Molecules Consisting Of: A hydrocarbon is a chemical compound made up only of carbon (C) and hydrogen (H) (H). The chemical framework is built up of carbon atoms, to which hydrogen atoms can be joined in a number of different ways. Both natural gas and petroleum are mostly composed of hydrocarbons in enormous quantities. These basic materials can be used as fuel and lubricant as well as converted into plastic, fiber, rubber, solvents, explosives, and industrial chemicals.
https://en.wikipedia.org/wiki/Hydrocarbon
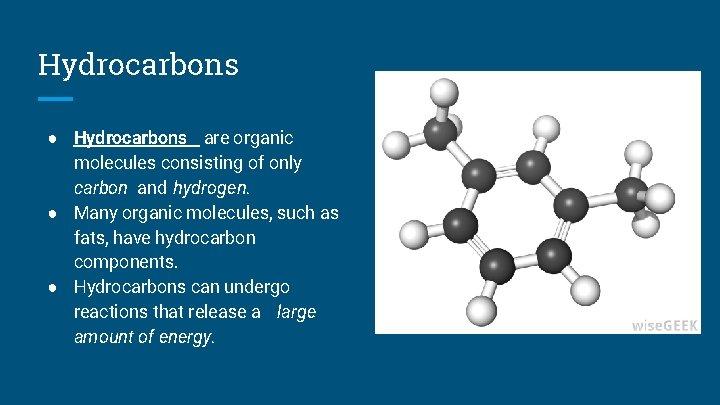
Numerous hydrocarbons can be found in nature. The pigment carotenes, which are also present in fossil fuels, trees, and plants, are present in foods like carrots and green leaves. Over 98 percent of natural crude rubber is a hydrocarbon polymer, which is a molecule composed of multiple linked units. Individual hydrocarbons’ chemistry and structure are significantly influenced by the chemical bonds that hold their atoms together in molecules.
Chemists in the nineteenth century classified hydrocarbons as either aromatic or aliphatic based on their sources and properties. Aliphatic hydrocarbons are hydrocarbons produced chemically as a result of the breakdown of fats or oils. Aromatic hydrocarbons, a class of related molecules, were created chemically through the degradation of specific plant extracts. In modern chemistry nomenclature, the molecules classified as aliphatic and aromatic compounds differ in structure rather than origin.
According to the kind of bonds they include, the three main groups of aliphatic hydrocarbons are alkanes, alkenes, and alkynes. Alkanes have a single bond, alkenes have a double bond, and alkynes have a triple bond. Aromatic hydrocarbons are defined as hydrocarbons with “exceptional stability,” meaning they are more stable than their Lewis structures would suggest. There are two types of aromatic hydrocarbons: arenes, which are stable because they contain a benzene ring, and nonbenzenoid aromatic hydrocarbons, which are less stable because they lack one.
Alkanes
Alkanes, which are saturated hydrocarbons, have just one covalent bond connecting the carbon atoms. In an alkane, the four-carbon or hydrogen atoms are each coupled to a different carbon atom by sp3 hybrid orbitals. Methane, ethane, and pentane’s Lewis structures and models are shown below. It’s crucial to keep in mind that Lewis structures are not intended to depict the dimensionality of molecules. As you can see, the structural illustrations of the pentane molecule do not contain any straight lines. An alkane takes on a zigzag shape when carbon chains with sp3 hybridization have bond angles close to 109.5°.

The terms “Hydrocarbon Chains” and “chains of hydrocarbons” are interchangeable
Carbon atoms can form branched or unbranched chains when they are joined together. The overall shape of the molecule is influenced by the several covalent bonds’ various geometries. For instance, it can be demonstrated that the various types of bonds that occur between carbon and carbon have an impact on the geometry of hydrocarbons, such as carbon-to-carbon bonds. All three molecules, which are two-carbon hydrocarbons, are named with the prefix “eth-.” The suffixes “–ane,” “–one,” and “–one,” respectively, indicate one, two, or three carbon-carbon bonds.
Accordingly, three-carbon molecules makeup propylene, propane, and propyne, while four carbon molecules make up butane, four-carbon molecules makeup butene, and so on. While double and triple bonds alter the form of the molecule, resulting in a planar or linear arrangement, single bonds can be rotated along the bond’s axis. These geometries have a significant impact on the shapes that a particular molecule can adopt.
When carbon forms single bonds with other atoms, the result is the formation of the tetrahedral structure of carbon. The shape that is created when two carbon atoms make a double bond together is planar, or flat. Single bonds in ethane have the capacity to rotate about their axes. The atoms on either side of double bonds are locked in place because they cannot rotate.
Rings made of Carbon
We have previously talked about aliphatic hydrocarbons, which are linear chains of carbon atoms. Another form of hydrocarbon with carbon atoms organized in a ring is an aromatic hydrocarbon. Due to the existence of two double bonds, the ring structure of cyclohexane can be likened to that of benzene. The benzene ring is present in certain amino acids, almost all steroids, cholesterol, and the hormones estrogen and testosterone. The benzene ring is also present in the herbicide 2,4-D. Benzene, a carcinogen, is a naturally occurring substance found in crude oil. An example of a hydrocarbon with both aliphatic and aromatic components is beta-carotene.
Hydrocarbons with an odor
Since it contains six carbon atoms, the benzene ring is exclusive to aromatic hydrocarbons. In terms of its physical and chemical characteristics, the benzene ring differs from alkanes due to the special energy features of its electrons. Because of their powerful scent, this group of compounds was previously known as aromatics. The characteristic properties of molecules with the label aromatic are today described by a six-membered ring, known as an aromatic ring.
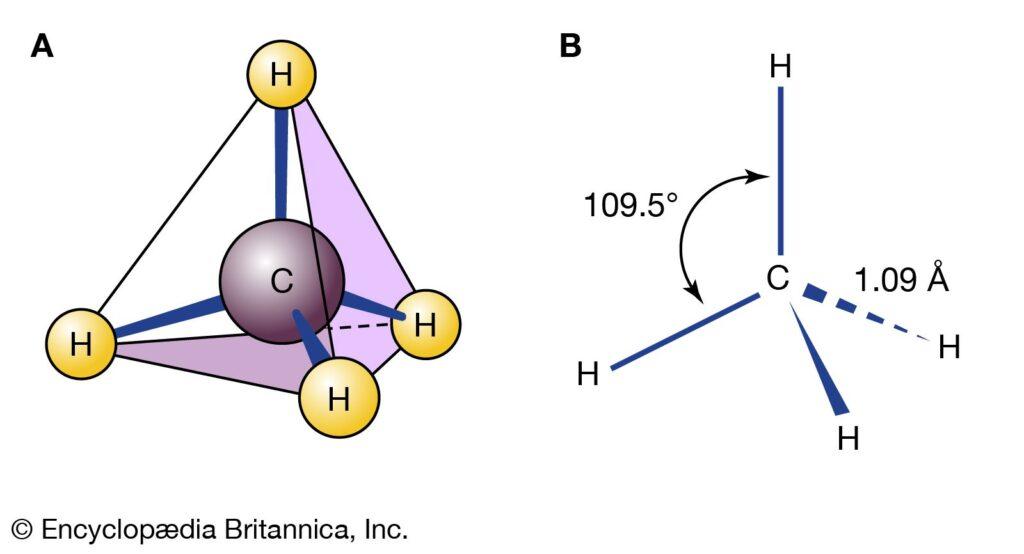
Regular alkanes are the most fundamental types of alkanes and have a straight chain of C atoms. Their names are derived from the number of C atoms in the chain. The smallest alkane is methane. Since the C atom is joined by four covalent bonds to four H atoms, the methane molecule has the chemical formula CH4. In the methane diagram, the C atom forms four covalent connections, but they are oriented three-dimensionally toward the corners of a square. Look no further than Figure 16.1 for a more precise representation of the methane molecule.
The following phrases are crucial in this context:
A chemical bond in which two or more electrons are shared by the two atoms is known as a covalent bond. An inorganic substance that has carbon arranged in an open-chain fashion. In an aromatic molecule, single and double bonds alternate while the ring’s electrons are spread out.
“Saturated Hydrocarbons”
The most fundamental organic molecule is methane, or CH4, which has one carbon atom and four hydrogen atoms. Gas burners in our houses generate a sizable amount of what is known as “natural gas,” which contains a sizable amount of this molecule. A completely saturated hydrocarbon is methane. These hydrocarbons are known as saturated hydrocarbons because there are only single bonds between the carbon atoms in them. Methane is the most elementary hydrocarbon, having only one carbon atom.
There’s always a chance that things could become a little more challenging. How can we determine whether a hydrocarbon has saturated? A hydrocarbon’s saturation can be assessed using the saturation formula and the chemical formula of the molecule. A saturated hydrocarbon contains 2N + 2 hydrogen atoms, which equals 4 carbon atoms. We must first calculate the number of carbon atoms in the molecule in order to use this formula (N in the formula).
Organic molecules and hydrocarbons are the two categories of organic molecules
Covalent bonds hold atoms together, and electron-pair bonds keep those bonds stable. Covalent bonds between carbon atoms enable the formation of organic molecules. These molecules include proteins, sugar, gasoline, and everything in between. Since carbon and hydrogen are two of the most often used atoms in organic chemistry, today’s discussion will center on molecules that include large amounts of these two components. The combination of hydrogen and carbon can result in a vast range of different compounds, all of which are referred to as hydrocarbons. Hydrocarbons can be divided into two categories: those that contain hydrogen and those that do not.
Important Points
Because only carbon and hydrogen are present in these molecules, they are known as hydrocarbons. Due to the unique bonding patterns of carbon, single, double, or triple bonds are possible. Through the act of bonding, hydrocarbons can create rings or chains.