Gelatinous Carbohydrate Obtained From: Gelatin manufacture is the most polluting industry process since it uses a large number of raw organic materials and chemicals (HCl, NaOH, Ca2+) as well as a large volume of end-pipe effluent. The biodegradability (BOD/COD ratio) of gelatinous wastewater (GWW) is more than 0.6. As a result, it represents a viable low-cost substrate for the anaerobic digestion process.
Which produces biofuels such as H2 and CH4. The anaerobic methods used for simultaneous treatment and energy recovery from GWW are thoroughly described in this paper. Protein concentration, organic loading rate (OLR), hydraulic retention time (HRT), the substrate to inoculum (S0/X0) ratio, type of mixed culture anaerobes, carbohydrates concentration, volatile fatty acids (VFAs), ammonia, and alkalinity/VFA ratio, and reactor configurations were all factors affecting biofuels productivity from anaerobic digestion of GWW. To assist further growth and achieve practicality in this domain, economic values and future perspectives that require more attention are also presented.
Gelatin production in the world ranges from 375,000 to 400,000 tonnes per year, making it a significant part of many countries’ economies. 300 m3 of gelatinous effluent is produced per tonne of raw materials, mostly bones. The gelatin industry produces a large amount of highly contaminated effluent that contains proteins, carbs, and lipids. The biological oxygen demand (BOD), chemical oxygen demand (COD), suspended solids (SS), and ammonia nitrogen (NH4-N) concentration of gelatin processing end-of-pipe effluents is high. Furthermore, the gelatinous wastewater (GWW) has high sulfate (SO42), calcium, and phosphorus concentrations.
The fundamental issue gelatinous wastewater
The main source of di-calcium phosphate is the demineralization of bones. The fundamental issue with gelatinous wastewater (GWW) is the odor, nuisance, and the fact that it is highly disagreeable to human habitation. Depending on the manufacturing process and the raw materials used, the GWW could be alkaline or acidic. GWW is a greasy, fibrous substance that contains bovine bone fragments and hairs, resulting in high levels of particulate organic matter. Animal pulverized bones, skin, and hair make up the majority of the particles, which is quite unpleasant.
The COD of GWW ranged from 9691.2 to 23,016 mg/L due to the use of protein, lipid, and carbohydrate-rich source materials. For 10–12 weeks, the bovine hide is soaked in a lime tank with a pH over 12.0, resulting in high calcium residual values in the effluent streams ranging from 3175.3 to 5210 mg/L and an alkaline pH of 11.1–12.4. During the manufacturing process, sodium hydroxide is added, resulting in an alkalinity of 5432–6527 mg CaCO3/L. The ammonification and acidification processes create sulfate (SO42) (1000–1496 mg/L) and NH4–N (163.2–190 mg/L) in the GWW.
The discharge of such effluent into sewerage systems or water streams pollutes drinking water severely. Because of its harmful influence on water streams, this effluent should be treated before being discharged into the environment. Simultaneous treatment and manufacture of biofuels from GWW, on the other hand, saves energy and chemicals while producing less surplus sludge.
Compositions, Properties, Manufacturing Processes, Uses, and Applications of Gelatin
Gelatin is a tasteless and odorless solid mass that is somewhat yellow, transparent, and brittle. Gelatin is primarily derived from the collagen found in the connective tissue of animals. Collagen accounts for 25–35 percent of total protein in an animal’s body. Gelatin is a protein with a high molecular weight that contains eighteen different amino acids. Proteins are made up of amino acids, which contain oxygen, carbon, nitrogen, and hydrogen. They have an amino (NH2) and a carboxyl (COOH–) group.
Gelatinous Carbohydrate Obtained From
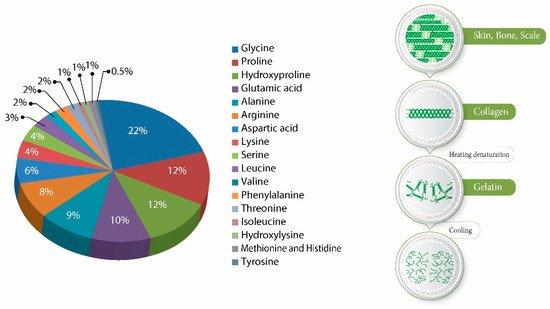
Gelatin has a protein content of 98–99 percent, which varies depending on the source of the original raw materials and the manufacturing procedure. Gelatin contains 22 percent glycine, 12 percent proline, 12 percent hydroxyproline, ten percent glutamic acid, nine percent alanine, eight percent arginine, six percent aspartic acid, four percent lysine, four percent serine, three percent leucine, two percent valine, two percent phenylalanine, two percent threonine, one percent isoleucine, one percent hydroxylysine, one percent methionine.
The procedures in the making of gelatin from animal bones and skin are depicted in the schematic figure. Pig and cattle skins are the most common sources of gelatin. To extract collagen, raw skin is dried, acidified, and then alkaline techniques are used. discusses the manufacturing techniques of gelatin derived from animals in detail. Observation of the Test Procedure In Molisch’s experiment a carbohydrate solution Is made in a- naphthol water with strong sulphuric acid poured along the side of the test tube. On the connection beneath the aqueous layer, a purple ring forms.
The test of Fehling To hydrolyze a polysaccharide, the sample substance (0.5 g) is heated with dilute hydrochloric acid. After adding sodium hydroxide solution to neutralize the reaction mixture, Fehling’s solutions 1 and 2 are added. Red reducing sugar precipitate (all monosaccharides, as well as numerous disaccharides such as lactose, maltose, cellobiose, and cellobiose) Benedict’s examination In a test tube, combine 0.5gm aqueous extract of plant material with 5ml Benedict’s solution and boil for 5 minutes. Then it’s let to cool down on its own.
A carbohydrate is a naturally occurring substance, or a derivative of such a compound, having the typical chemical formula Cx(H2O)y, composed of carbon (C), hydrogen (H), and oxygen (O) molecules in a proportion similar to that of water. • Carbohydrates are the most common organic compounds and are essential to all living. • Carbohydrates are aldehyde or ketone alcohols that are polyhydroxy aldehydes, polyhydroxy ketones, or molecules that can be hydrolyzed to them chemically. • Carbohydrates are named carbohydrates because they are essentially carbon hydrates (i.e. they are made up of carbon and water and have a chemical formula of (CH2O)n.
Sustainable Solutions (GWW)
Simultaneous Treatment and Energy Recovery from Gelatinous Wastewater: To treat GWW, several treatment techniques were used, including the coagulation process, which removed just 50% of total organic carbon (TOC). The effluent quality does not meet discharge requirements. The efficacy of electrocoagulation and electrooxidation for the treatment of GWW was also explored by the authors. They discovered that electrocoagulation with aluminum as an anode only enhanced TOC removal effectiveness by 10%.
These technologies use a lot of energy and chemicals, create a lot of sludge, and require specialized operation and maintenance expertise. Fortunately, GWW has a high biodegradability and can be used to produce biofuels (H2 and CH4) by anaerobic digestion. Carbohydrates and their derivatives: Sugars and medications that contain sugar. Sucrose, dextrose, glucose, fructose, and other sugars are examples. Polysaccharides and polysaccharide-containing medicines, starches, dextrins, and other polysaccharide-containing substances tragacanth, acacia, sterculia, sodium, alginate, agar, and cellulose are all examples of gums and mucilages.
The word saccharide comes from the Greek word saccharin, which means “sugar.” Monosaccharides are sugars that cannot be digested into simpler sugars, such as D-glucose (commonly known as dextrose). When disaccharides are hydrolyzed, they yield two monosaccharide molecules (e.g. sucrose, which is hydrolyzed into glucose and fructose) On hydrolysis, trisaccharides generate 3 monosaccharides molecules, while tetrasaccharides yield 4 monosaccharide molecules, and so on.
The number of carbon atoms in a molecule determines the number of carbon atoms in the molecule. Monosaccharides include trioses with three carbon atoms, tetroses with four carbon atoms, pentoses with five carbon atoms, and hexoses with six carbon atoms, among others. Oligosaccharides are carbohydrates that are made up of a few monosaccharide units connected together by oxygen.




